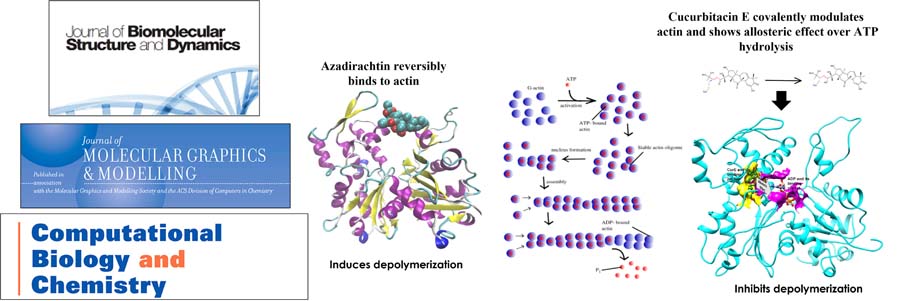
Mechanical understanding of the correlation between actin assembly and ATP hydrolysis has been an object of intensive studies in biochemistry and structural biology. Azadirachtin(A) (AZA), a potential insecticide from neem, binds to actin and induces depolymerization in Drosophila. AZA binds to the pocket the same as that of Latrunculin A (LAT), but LAT inhibits actin polymerization by stiffening the actin structure and affects the ADP-ATP exchange. Cucurbitacin E (CurE) modulates the actin cytoskeleton by forming an irreversible covalent bond with Cys257 of actin. The reported binding conformation of CurE is deeply buried in the subdomain 4 of actin and is closely situated to the ATP-binding site. CurE allosterically modulates ADP and stabilizes the F-actin structure, affecting the nucleotide exchange and depolymerization of F-actin.
References:
1. Roopa, L., Akshai, P. S., & Pravin Kumar, R. (2019). Connecting the dots in the mechanism of action of Cucurbitacin E (CurE) – path analysis and steered molecular dynamics reveal the precise site of entry and the passage of CurE in filamentous actin. Journal of Biomolecular Structure and Dynamics, 38(3), 635–646. https://doi.org/10.1080/07391102.2019.1593243
2. Kumar, R. P., Roopa, L., Nongthomba, U., Sudheer Mohammed, M. M., & Kulkarni, N. (2016). Docking, molecular dynamics, and QM/MM studies delineate the mode of binding of CucurbitacinE to F-actin. Journal of Molecular Graphics and Modelling, 63, 29–37. https://doi.org/10.1016/j.jmgm.2015.11.007
3. L, R., R, P. K., & M.M., S. M. (2016). Molecular dynamics and high throughput binding free energy calculation of anti-actin anticancer drugs—New insights for better design. Computational Biology and Chemistry, 64, 47–55. https://doi.org/10.1016/j.compbiolchem.2016.05.008
4. Kumar, R. P., Roopa, L., Mohammed, M. M., & Kulkarni, N. (2015). Azadirachtin(A) Distinctively Modulates Subdomain 2 of Actin – Novel Mechanism to Induce Depolymerization revealed by Molecular Dynamics Study. Journal of Biomolecular Structure and Dynamics, 1–39. https://doi.org/10.1080/07391102.2015.1127665