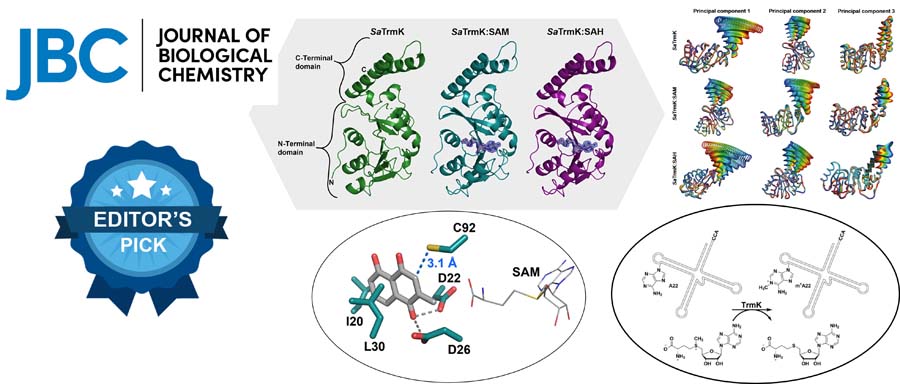
The enzyme m1A22-tRNA methyltransferase (TrmK) from Staphylococcus aureus catalyzes the transfer of a methyl group to the N1 of adenine 22 in bacterial tRNAs. TrmK is essential for S. aureus survival during infection, but has no homolog in mammals, making it a promising target for antibiotic development.
Highlights
- Molecular dynamics simulations point to specific motions of the C-terminal domain being altered by SAM binding, which might have implications for tRNA recruitment
- In-silico screening revealed Plumbagin as a potential inhibitor of TrmK
- Plumbagin is a Michael acceptor and can undergo conjugate addition with amino and thiolate groups on its C3 position. It inhibits SaTrmK via covalent attachment to Cys92 to form a Michael adduct