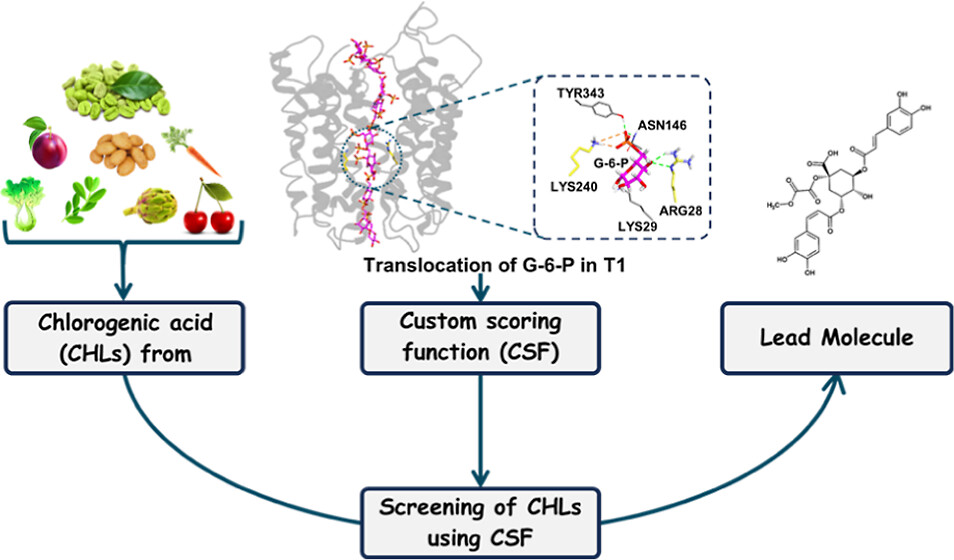
Chlorogenic acids (CHLs) are known to competitively bind to translocase-I (T1) of the glucose-6-phosphatase (G6 Pase) system, thereby inhibiting the transport of glucose-6-phosphate (G6P). This competitive binding results in a consequential reduction in blood sugar levels. In this study, steered molecular dynamics (SMD) simulation is employed to investigate the interaction between T1 and G6P, aiming to gain insights into the binding dynamics and diffusion process of G6P through T1. A database comprising 41 CHLs sourced from various plants was developed, subjected to minimization, and screened against T1 through conventional docking methods. The docked conformations were fed into a newly developed customized scoring method incorporating contact-based weights to assess the binding affinities that systematically rank and identify the most effective competitive inhibitors. Among the screened CHLs, 1-methoxy 3,5-dicaffeoylquinic acid, 3,4 dicaffeoyl quinic acid, and 3,4,5-tricaffeoylquinic acid stood out as the top three inhibitors, showcasing crucial atomic interactions with key residues within the binding pocket of T1, and these CHLs are sourced from readily available plants, diminishing reliance on coffee as the predominant CHL source. Along with the devised scoring function, which serves as a valuable tool for virtual screening and lead optimization in drug development, this study also marks a pioneering effort as it involves the modeling of the human translocase and unravels the mechanism of binding and diffusion of G6P within human T1, providing valuable insights into the structural prerequisites for successfully inhibiting the G6P system, laying the foundation for a rational approach to drug design. This research contributes to the progress of drug discovery strategies focused on the G6P system, presenting potential therapeutic avenues for addressing metabolic disorders linked to an impaired glucose metabolism.
In Silico Screening of Chlorogenic Acids from Plant Sources against Human Translocase-I to Identify Competitive Inhibitors to Treat Diabetes
October 17, 2024by Kcat Editor